
Cynthia A. Janak
New Gardasil findings about clinical trials
By Cynthia A. Janak
In my article Gardasil Clinical Trials — Placebo that I wrote July 23, 2008 I showed you Table 7. In that table it referenced Study 018 and that this study had a saline placebo.
Being the truthful person I am, I am going to admit that this study, in fact, did not have a saline placebo. I have finally had the chance to sit down and read the whole 464 page report to the FDA (http://www.fda.gov/cber/review/hpvmer060806r.pdf).
I have found starting on page 300, 8.1.6: Trial #6, Protocol 018 Objectives, Design: bullet point three that the saline placebo stated in Table 7 is false. In fact it was a non-aluminum containing placebo.
Design:
• Randomized, double-blind, placebo controlled, multicenter study in 9-15 year old subjects.
• Enrollment was stratified by age and gender. Subjects were to be enrolled into 2 age strata (9 to 12 years of age and 13 to 15 years of age) in approximately a 2:1 ratio.
• The ratio of enrolled boys to girls was to be approximately 1:1. Approximately 1650 subjects were to be randomized in a 2:1 ratio to receive either quadrivalent HPV (Types 6, 11, 16, 18) L1 VLP vaccine or nonaluminum-containing placebo. Randomization was stratified by study center only.
When I read this it brought back the memory of the conversation that I had in September with one of the lead scientists in the Brazilian study. I asked this person why they did not use a saline placebo in the studies. Their answer to me was, "Why should we use a saline placebo? The only side-effect from a saline placebo would be injection side pain." I had thought that this person was only speaking of the study that they were a part of, not the whole study. That is where my mistake happened.
So at this juncture I must apologize for this error on my part and I am going to correct that error now.
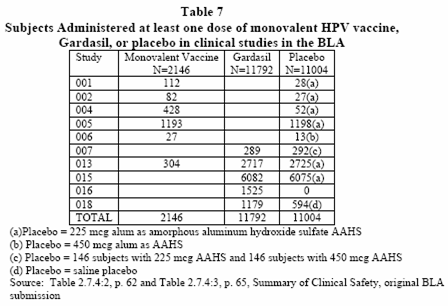
As you can see table 7, under study group 018, has participants of 1179 for the Gardasil group and 594(d) for the Placebo group. Below this study it has the description of what the (d) means. This shows that the (d) is a Placebo = saline placebo.
Looking at this table you would think that this would prove the safety of the vaccine. You would think that you would get a reasonable comparison of the differences when you add all the ingredients that go into Gardasil. That is my assumption anyway.
Starting with page 300 of this report is where you will find the details of study 018. The first table in this section is table 209 and 210.
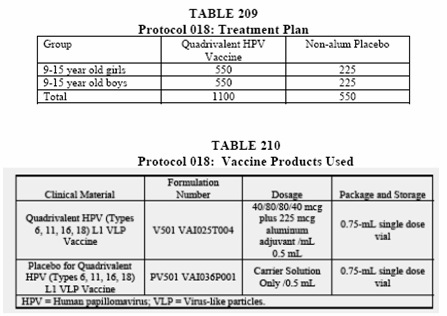
As you can see they mention a "non-alum placebo" and a "carrier solution" placebo.
Population: Protocol 018 was conducted in 47 sites in 10 countries in North America (US), Latin America (Colombia, Mexico), Europe (UK, Portugal, Norway, Denmark, Spain) and Asia (Thailand, Taiwan). The subjects were to be healthy preadolescents and adolescents who are not sexually active.
For full Inclusion and Exclusion Criteria, see APPENDIX 17
Vaccination schedule: Subjects received vaccine or placebo (0.5 mL) IM at 0, 2, and 6 months.
Concomitant Vaccines: None planned.
Endpoints
Primary Immunogenicity Endpoints
• Anti-HPV 6, 11, 16, 18 GMTs Week 4 postdose 3
• Pecentage of subjects who seroconverted (change in serostatus from seronegative to seropositive) for each of the vaccine HPV types by Week 4 postdose 3. Seropositive is defined as anti-HPV serum cLIA levels 20, 16, 20, 24 mMU/mL for HPV types 6, 11, 16, and 18, respectively.
Primary Safety Endpoints
• Occurrence of severe injection site AEs
• Incidence of any VR related SAE
On page 302 of this document they have a section called "special Procedures." Under this heading, the first bullet point, they state something interesting.
Because the true placebo was visually distinguishable from the HPV vaccine, ...
How can they say a "true placebo" when that was not the case with study 018? The fact is that the placebo was a "carrier solution" so this statement is also false and misleading.
Safety Objectives
• In order to address this objective, the study called for a detailed tolerability analysis, with emphasis on the following prespecified adverse experiences: vaccine-related adverse experiences, VRC-prompted injection-site adverse experiences (swelling/redness and pain/tenderness/soreness), VRC-prompted systemic adverse experiences (muscle/joint pain, headaches, hives, rashes, diarrhea), severe adverse experiences, and fever. Risk differences were calculated for AEs comparing the vaccine and placebo groups across all vaccination visits with respect to all AEs with > 1% incidences. p-values were computed for VRC elicited adverse events only
• Adverse experiences were summarized descriptively as frequencies and percentages by vaccination group and type of adverse experience, by vaccination visit and across all vaccination visits.
• Risk differences and associated exact 95% confidence intervals were computed comparing the vaccine and placebo groups across all vaccination visits with respect to adverse experiences with 1% incidence in either vaccination group.
Then on page 303 they make these statements about the safety objectives. Basically, they are stating that the adverse events between the two groups are less than one percent. Of course, there would not be a great difference because the placebo was not a true saline placebo. This is what they are basing their safety data on, the difference between the two shots. Not the fact that there were adverse events with both shots that to me would make it an unsafe vaccine.
Now I want to go to page 316 where you find the "Safety Results." This is what they say about this.
Safety Results
• Overall, a higher proportion of vaccine recipients reported one or more AEs compared with placebo recipients. This was largely due to a higher proportion of vaccine recipients with injection site AEs compared to placebo recipients. The proportion of subjects with systemic AEs was comparable in the 2 groups.
• Overall, 5 SAEs occurred within the 14 days after any vaccination. All of these occurred after receipt of the vaccine and none after placebo. (These are detailed later in the review).
• 2 subjects discontinued due to an AE (both in the vaccine group). (These are detailed later in the review).
• The AEs postdose 1, 2, and 3 were generally consistent with those described for the overall clinical AE summary. Higher proportions of subjects in the vaccine and placebo reported AEs (overall, injection site, and systemic AE) following dose 1 compared with postdoses 2 and 3. (Source: Tables 11-26, 11-27, 11-28, CSR 018v1, p. 254-6, not shown here)
• The AEs in the 9-12 year olds and 13-15 year olds were generally comparable to the overall AE summary. 4/5 of the SAEs occurred in the 13-15 year olds.
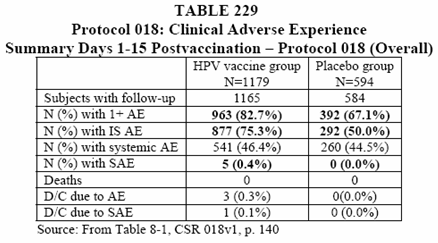
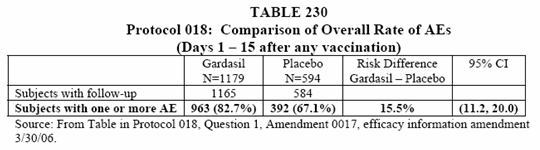
• There was a statistically higher proportion of subjects in the vaccine group with an AE as compared to the saline placebo group. (See Table 230 below).
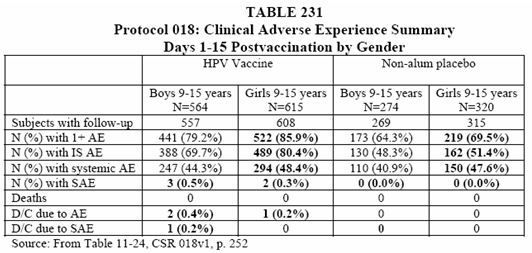
• In each vaccination group, a higher proportion of girls reported an AE compared with the boys. (See Table 231 below).
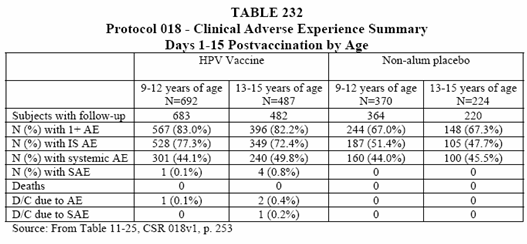
• The overall proportions of subjects with an AE were similar when comparing the 9-12 year old subjects with the 13-15 year old subjects who received vaccine. The 13-15 year old vaccine recipients had a lower overall proportion of subjects with an injection site AE as compared to 9-12 year old vaccine recipients (with the same pattern noted for placebo). The 13-15 year old vaccine recipients had a slightly higher proportion of subjects with systemic AEs as compared to the 9-12 year old vaccine recipients. A similar proportion of 9-12 year old vaccine recipients had systemic AEs as compared to 9-12 year old placebo recipients. (See Table 232 below).
Hey, it gets better. Now let us look at page 318, section called "Intensities of AEs" (adverse events).
Intensities of AEs
• The proportions of subjects reporting a moderate or severe injection site AE (of all subjects with follow-up data) were higher in the quadrivalent vaccine group (32.5% for moderate, and 10.6% for severe) as compared to the non-alum placebo group (23.6% for moderate and 6.8% for severe).
• Among all reported AEs, the frequency of intensity ratings appeared comparable between the 2 vaccination groups.
• There were 3 AEs reported per vaccine recipient, and 2 AEs reported per placebo recipient. (Source: Tables 8-2, and 8-3, CSR 018v1, p. 143, not shown here)
I want you to take note that they decided not to show this table on this report. I would think that this would be important information in regards to the safety of this vaccine but I guess the person who put this report together from the FDA did not feel that her cohorts needed to know this information.
Now let us go on to the section titled "Injection Site AEs." I want you to note that they also mention in these tables the non-alum placebo.
Injection Site AEs
• In both vaccination groups, the most common injection site AE was pain in the 5 days after any vaccination. (See Table 233 below).

• Significantly higher proportions of vaccine recipients reported injection site erythema, pain and swelling as compared to placebo recipients. Risk differences were compared. (See Table 234 below.)

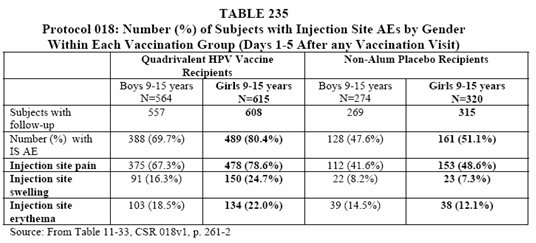
• The proportions of subjects with injection site AEs within each gender group were generally comparable to those observed in the study overall. In the vaccine group, the proportion of girls who reported one or more injection site AE (80.4%) was higher than the proportion of boys with one or more injection site AE (69.7%). (See Table 235 below).
• The proportion of subjects with specific injection site AEs within each age stratum are provided in Table 236 below. Injection site pain, swelling and erythema were reported in a higher proportion of vaccine recipients 9-12 years of age as compared to vaccine recipients 13-15 years of age.
This is getting really long so I am going to cut to the chase. This is the most disturbing part of this report in regards to study 018. The creator of this report did not feel that it was important to give the reviewers of the safety protocol for this vaccine this detailed information about "New Medical Conditions" section directly below.
New Medical Conditions
• The proportions of subjects with new medical conditions through Month 7 were comparable between the vaccination and placebo groups.
• The most common new medical conditions during this time period were headache and URI. (Source: Table 8-24, CSR 018v1, p. 181-2, not shown here)
• There was one case of autoimmune thyroiditis in the vaccine group and none in the placebo group. The sponsor provided additional information about this condition in general and across studies. A discussion is included in the safety summary in this document. (Source: Table 11-79, CSR 018v1, p. 354-63, not shown here)
Follow-up from Month 7 to Month 12
• Subjects (guardians) were contacted at Month 12 of the study to assess for any new medical conditions.
• Overall, 95.0% of subjects randomized (95.3% Gardasil group and 94.4% of placebo group) entered the Persistence Phase of Protocol 018. Five subjects discontinued from the study (3 in the vaccine group: 2 lost to follow-up and 1 withdrew consent; 2 in the placebo group due to relocation).
• A lower proportion of vaccine recipients (29.0%) reported a new medical condition between Day 1 and Month 12 as compared to placebo recipients (31.0%). This was also noted in new medical conditions between Month 7 and Month 12. See Tables 245 and 246 below).
• There were no new SAEs reported in this period.

Comments-Conclusion Regarding Data for Protocol 018 (Reviewer's Opinion)
• Protocol 018 provides saline placebo-controlled safety data for subjects 9-15 years of age (617 girls and 567 boys who received vaccine). This is of particular interest because the other studies used alum placebo as a safety comparison.
As you can see tables 245 and 246 just say placebo. Someone scanning through the 464 pages of this document like I did initially would be led to believe that this was referring the saline placebo. In essence this is the non-alum carrier solution placebo that was used.
The "Reviewer's Opinion" falsely claims that this protocol 018 used a saline placebo. As I have shown you that placebo was in reality a carrier solution placebo.
Nowhere in the Gardasil trials did they ever, ever use a saline placebo. The generator of this report led the FDA to believe that they did in one little study and that is false.
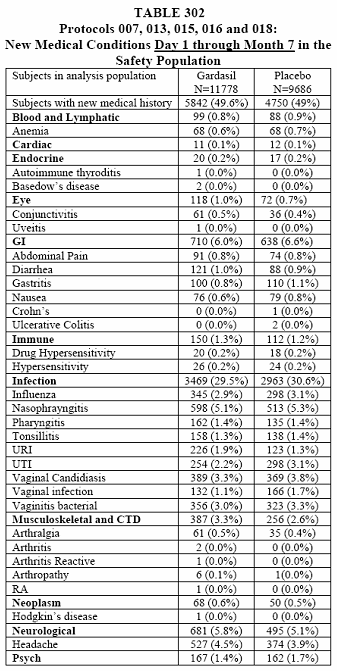
The piece de resistance is the cumulative tables of the new medical conditions for all study groups.
I want you to note in the last tables, 302 and 303, that these are only of the most common new medical conditions and those of interest (with slight inequality between vaccine and placebo). This was taken from page 392, 10.3.5 New Medical History.
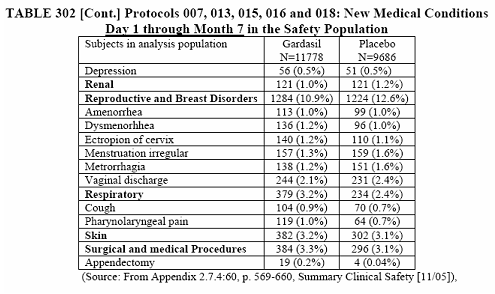
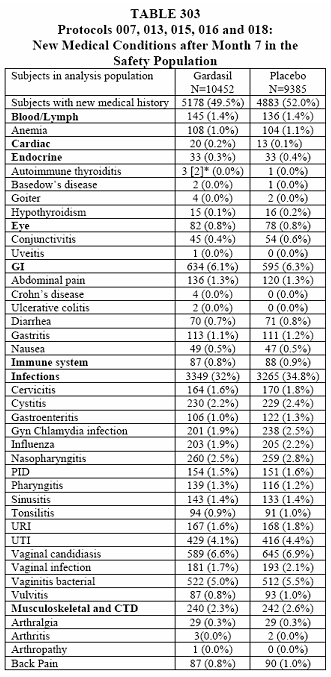
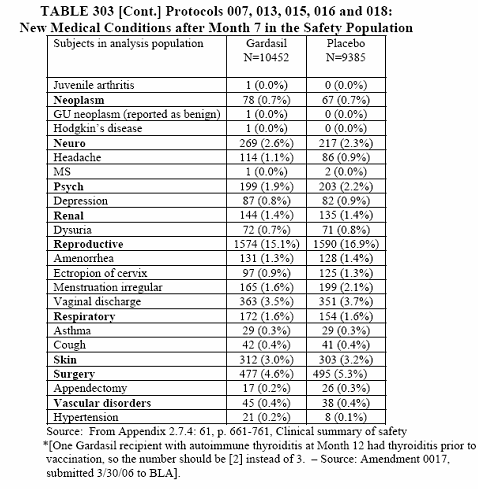
To summarize this long and detailed article you have a better than 50% chance of having a new medical condition after receiving the Gardasil vaccine. At least those odds are better than the report of September 11, 2008 which is 73.3% chance of acquiring a new medical condition. The only problem is that I have to base this percentage on the most common new medical conditions and those of interest. The actual percentage when everything is taken into consideration can be as high as the report of September 11, 2008. We just do not know because this reporter decided that it was not important.
What all this means is that in the United States you have the real possibility of 2,902,164 girls that have a new medical condition. This is better than the numbers that I gave you in my last article because I based them on the results of the report of September 11, 2008.
The other item is that Gardasil never had a placebo group in any of their studies and that the reporter that generated this document used false statements in regards to the saline placebo. The, so called, saline placebo was in reality a non-alum carrier solution placebo.
I would suggest that the FDA looks at this new information and that they pull this vaccine off the market due to the fact that there is a good chance that 50% of the girls vaccinated with Gardasil will have a new medical condition that can alter their standard of living dramatically and that it could increase the number of the disabled in this country.
The same recommendation goes to all the countries that are administering either of the HPV vaccines. The odds of 50% to 73.3% are too high in my opinion to continue with this type of vaccination.
For more information about Cervarix and Gardasil please visit my website at www.cynthiajanak.com
© Cynthia A. Janak
December 8, 2008
In my article Gardasil Clinical Trials — Placebo that I wrote July 23, 2008 I showed you Table 7. In that table it referenced Study 018 and that this study had a saline placebo.
Being the truthful person I am, I am going to admit that this study, in fact, did not have a saline placebo. I have finally had the chance to sit down and read the whole 464 page report to the FDA (http://www.fda.gov/cber/review/hpvmer060806r.pdf).
I have found starting on page 300, 8.1.6: Trial #6, Protocol 018 Objectives, Design: bullet point three that the saline placebo stated in Table 7 is false. In fact it was a non-aluminum containing placebo.
Design:
• Randomized, double-blind, placebo controlled, multicenter study in 9-15 year old subjects.
• Enrollment was stratified by age and gender. Subjects were to be enrolled into 2 age strata (9 to 12 years of age and 13 to 15 years of age) in approximately a 2:1 ratio.
• The ratio of enrolled boys to girls was to be approximately 1:1. Approximately 1650 subjects were to be randomized in a 2:1 ratio to receive either quadrivalent HPV (Types 6, 11, 16, 18) L1 VLP vaccine or nonaluminum-containing placebo. Randomization was stratified by study center only.
When I read this it brought back the memory of the conversation that I had in September with one of the lead scientists in the Brazilian study. I asked this person why they did not use a saline placebo in the studies. Their answer to me was, "Why should we use a saline placebo? The only side-effect from a saline placebo would be injection side pain." I had thought that this person was only speaking of the study that they were a part of, not the whole study. That is where my mistake happened.
So at this juncture I must apologize for this error on my part and I am going to correct that error now.

As you can see table 7, under study group 018, has participants of 1179 for the Gardasil group and 594(d) for the Placebo group. Below this study it has the description of what the (d) means. This shows that the (d) is a Placebo = saline placebo.
Looking at this table you would think that this would prove the safety of the vaccine. You would think that you would get a reasonable comparison of the differences when you add all the ingredients that go into Gardasil. That is my assumption anyway.
Starting with page 300 of this report is where you will find the details of study 018. The first table in this section is table 209 and 210.

As you can see they mention a "non-alum placebo" and a "carrier solution" placebo.
Population: Protocol 018 was conducted in 47 sites in 10 countries in North America (US), Latin America (Colombia, Mexico), Europe (UK, Portugal, Norway, Denmark, Spain) and Asia (Thailand, Taiwan). The subjects were to be healthy preadolescents and adolescents who are not sexually active.
For full Inclusion and Exclusion Criteria, see APPENDIX 17
Vaccination schedule: Subjects received vaccine or placebo (0.5 mL) IM at 0, 2, and 6 months.
Concomitant Vaccines: None planned.
Endpoints
Primary Immunogenicity Endpoints
• Anti-HPV 6, 11, 16, 18 GMTs Week 4 postdose 3
• Pecentage of subjects who seroconverted (change in serostatus from seronegative to seropositive) for each of the vaccine HPV types by Week 4 postdose 3. Seropositive is defined as anti-HPV serum cLIA levels 20, 16, 20, 24 mMU/mL for HPV types 6, 11, 16, and 18, respectively.
Primary Safety Endpoints
• Occurrence of severe injection site AEs
• Incidence of any VR related SAE
On page 302 of this document they have a section called "special Procedures." Under this heading, the first bullet point, they state something interesting.
Because the true placebo was visually distinguishable from the HPV vaccine, ...
How can they say a "true placebo" when that was not the case with study 018? The fact is that the placebo was a "carrier solution" so this statement is also false and misleading.
Safety Objectives
• In order to address this objective, the study called for a detailed tolerability analysis, with emphasis on the following prespecified adverse experiences: vaccine-related adverse experiences, VRC-prompted injection-site adverse experiences (swelling/redness and pain/tenderness/soreness), VRC-prompted systemic adverse experiences (muscle/joint pain, headaches, hives, rashes, diarrhea), severe adverse experiences, and fever. Risk differences were calculated for AEs comparing the vaccine and placebo groups across all vaccination visits with respect to all AEs with > 1% incidences. p-values were computed for VRC elicited adverse events only
• Adverse experiences were summarized descriptively as frequencies and percentages by vaccination group and type of adverse experience, by vaccination visit and across all vaccination visits.
• Risk differences and associated exact 95% confidence intervals were computed comparing the vaccine and placebo groups across all vaccination visits with respect to adverse experiences with 1% incidence in either vaccination group.
Then on page 303 they make these statements about the safety objectives. Basically, they are stating that the adverse events between the two groups are less than one percent. Of course, there would not be a great difference because the placebo was not a true saline placebo. This is what they are basing their safety data on, the difference between the two shots. Not the fact that there were adverse events with both shots that to me would make it an unsafe vaccine.
Now I want to go to page 316 where you find the "Safety Results." This is what they say about this.
Safety Results
• Overall, a higher proportion of vaccine recipients reported one or more AEs compared with placebo recipients. This was largely due to a higher proportion of vaccine recipients with injection site AEs compared to placebo recipients. The proportion of subjects with systemic AEs was comparable in the 2 groups.
• Overall, 5 SAEs occurred within the 14 days after any vaccination. All of these occurred after receipt of the vaccine and none after placebo. (These are detailed later in the review).
• 2 subjects discontinued due to an AE (both in the vaccine group). (These are detailed later in the review).
• The AEs postdose 1, 2, and 3 were generally consistent with those described for the overall clinical AE summary. Higher proportions of subjects in the vaccine and placebo reported AEs (overall, injection site, and systemic AE) following dose 1 compared with postdoses 2 and 3. (Source: Tables 11-26, 11-27, 11-28, CSR 018v1, p. 254-6, not shown here)
• The AEs in the 9-12 year olds and 13-15 year olds were generally comparable to the overall AE summary. 4/5 of the SAEs occurred in the 13-15 year olds.

• There was a statistically higher proportion of subjects in the vaccine group with an AE as compared to the saline placebo group. (See Table 230 below).

• In each vaccination group, a higher proportion of girls reported an AE compared with the boys. (See Table 231 below).

• The overall proportions of subjects with an AE were similar when comparing the 9-12 year old subjects with the 13-15 year old subjects who received vaccine. The 13-15 year old vaccine recipients had a lower overall proportion of subjects with an injection site AE as compared to 9-12 year old vaccine recipients (with the same pattern noted for placebo). The 13-15 year old vaccine recipients had a slightly higher proportion of subjects with systemic AEs as compared to the 9-12 year old vaccine recipients. A similar proportion of 9-12 year old vaccine recipients had systemic AEs as compared to 9-12 year old placebo recipients. (See Table 232 below).

Hey, it gets better. Now let us look at page 318, section called "Intensities of AEs" (adverse events).
Intensities of AEs
• The proportions of subjects reporting a moderate or severe injection site AE (of all subjects with follow-up data) were higher in the quadrivalent vaccine group (32.5% for moderate, and 10.6% for severe) as compared to the non-alum placebo group (23.6% for moderate and 6.8% for severe).
• Among all reported AEs, the frequency of intensity ratings appeared comparable between the 2 vaccination groups.
• There were 3 AEs reported per vaccine recipient, and 2 AEs reported per placebo recipient. (Source: Tables 8-2, and 8-3, CSR 018v1, p. 143, not shown here)
I want you to take note that they decided not to show this table on this report. I would think that this would be important information in regards to the safety of this vaccine but I guess the person who put this report together from the FDA did not feel that her cohorts needed to know this information.
Now let us go on to the section titled "Injection Site AEs." I want you to note that they also mention in these tables the non-alum placebo.
Injection Site AEs
• In both vaccination groups, the most common injection site AE was pain in the 5 days after any vaccination. (See Table 233 below).

• Significantly higher proportions of vaccine recipients reported injection site erythema, pain and swelling as compared to placebo recipients. Risk differences were compared. (See Table 234 below.)

• The proportions of subjects with injection site AEs within each gender group were generally comparable to those observed in the study overall. In the vaccine group, the proportion of girls who reported one or more injection site AE (80.4%) was higher than the proportion of boys with one or more injection site AE (69.7%). (See Table 235 below).

• The proportion of subjects with specific injection site AEs within each age stratum are provided in Table 236 below. Injection site pain, swelling and erythema were reported in a higher proportion of vaccine recipients 9-12 years of age as compared to vaccine recipients 13-15 years of age.
This is getting really long so I am going to cut to the chase. This is the most disturbing part of this report in regards to study 018. The creator of this report did not feel that it was important to give the reviewers of the safety protocol for this vaccine this detailed information about "New Medical Conditions" section directly below.
New Medical Conditions
• The proportions of subjects with new medical conditions through Month 7 were comparable between the vaccination and placebo groups.
• The most common new medical conditions during this time period were headache and URI. (Source: Table 8-24, CSR 018v1, p. 181-2, not shown here)
• There was one case of autoimmune thyroiditis in the vaccine group and none in the placebo group. The sponsor provided additional information about this condition in general and across studies. A discussion is included in the safety summary in this document. (Source: Table 11-79, CSR 018v1, p. 354-63, not shown here)
Follow-up from Month 7 to Month 12
• Subjects (guardians) were contacted at Month 12 of the study to assess for any new medical conditions.
• Overall, 95.0% of subjects randomized (95.3% Gardasil group and 94.4% of placebo group) entered the Persistence Phase of Protocol 018. Five subjects discontinued from the study (3 in the vaccine group: 2 lost to follow-up and 1 withdrew consent; 2 in the placebo group due to relocation).
• A lower proportion of vaccine recipients (29.0%) reported a new medical condition between Day 1 and Month 12 as compared to placebo recipients (31.0%). This was also noted in new medical conditions between Month 7 and Month 12. See Tables 245 and 246 below).
• There were no new SAEs reported in this period.

Comments-Conclusion Regarding Data for Protocol 018 (Reviewer's Opinion)
• Protocol 018 provides saline placebo-controlled safety data for subjects 9-15 years of age (617 girls and 567 boys who received vaccine). This is of particular interest because the other studies used alum placebo as a safety comparison.
As you can see tables 245 and 246 just say placebo. Someone scanning through the 464 pages of this document like I did initially would be led to believe that this was referring the saline placebo. In essence this is the non-alum carrier solution placebo that was used.
The "Reviewer's Opinion" falsely claims that this protocol 018 used a saline placebo. As I have shown you that placebo was in reality a carrier solution placebo.
Nowhere in the Gardasil trials did they ever, ever use a saline placebo. The generator of this report led the FDA to believe that they did in one little study and that is false.
The piece de resistance is the cumulative tables of the new medical conditions for all study groups.
I want you to note in the last tables, 302 and 303, that these are only of the most common new medical conditions and those of interest (with slight inequality between vaccine and placebo). This was taken from page 392, 10.3.5 New Medical History.




To summarize this long and detailed article you have a better than 50% chance of having a new medical condition after receiving the Gardasil vaccine. At least those odds are better than the report of September 11, 2008 which is 73.3% chance of acquiring a new medical condition. The only problem is that I have to base this percentage on the most common new medical conditions and those of interest. The actual percentage when everything is taken into consideration can be as high as the report of September 11, 2008. We just do not know because this reporter decided that it was not important.
What all this means is that in the United States you have the real possibility of 2,902,164 girls that have a new medical condition. This is better than the numbers that I gave you in my last article because I based them on the results of the report of September 11, 2008.
The other item is that Gardasil never had a placebo group in any of their studies and that the reporter that generated this document used false statements in regards to the saline placebo. The, so called, saline placebo was in reality a non-alum carrier solution placebo.
I would suggest that the FDA looks at this new information and that they pull this vaccine off the market due to the fact that there is a good chance that 50% of the girls vaccinated with Gardasil will have a new medical condition that can alter their standard of living dramatically and that it could increase the number of the disabled in this country.
The same recommendation goes to all the countries that are administering either of the HPV vaccines. The odds of 50% to 73.3% are too high in my opinion to continue with this type of vaccination.
For more information about Cervarix and Gardasil please visit my website at www.cynthiajanak.com
© Cynthia A. Janak
The views expressed by RenewAmerica columnists are their own and do not necessarily reflect the position of RenewAmerica or its affiliates.
(See RenewAmerica's publishing standards.)





















